1. Characterisation of silico-alumino-phosphate molecular sieves (SAPO). Use of forcefield simulations intended to understand the catalytic properties of these materials.
Initially, the structure of these (crystalline) materials can be considered as an AlPO (alumino-phosphate) framework... Some considerations about AlPOs...
AlPO frameworks contain tetrahedrically coordinated Al and P (TO4 units, where T=Al,P), edged linked forming -T1-O-T2- units in which there is a strict structural alternance between Al & P: that means if T1=Al, then T2=P; or to put it another way the first T environments of Al and P are: P(4Al), and Al(4P). This fact is frequently refered to as 'ordered material', because no Al-O-Al, or P-O-P ever appear, as it should happen in a 'random' distribution for Al & P in the network. This has important consequences in the configurational entropy of the material about which I can tell you privately because I don't want to bother you too much. continued...
2. Diffusion of C8 aromatics in a 10 and 12 Membered Rings Zeolite (CIT-1).
Use of forcefield Molecular Dynamics aimed to calculate self-diffusivities, activation energies, and diffusion features: shape selectivity effects caused by the two-channel microporous structure and the different size of the guest molecules.
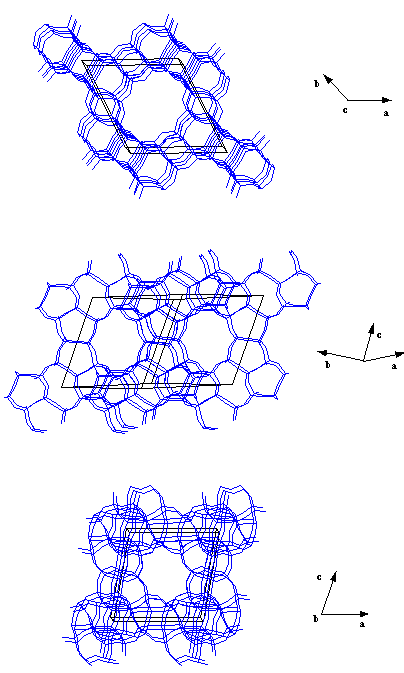
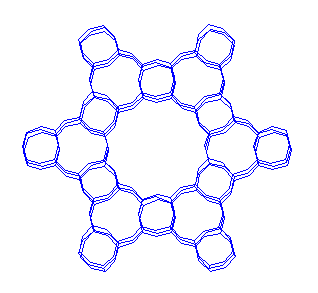
It is well known that two of the most iportant properties of zeolites are their microporosity and acidity. The microporososity of these crystalline materials is controlled in the synthesis process according to the pore and channel size distribution of the structure. Channels are window openings formed by 4,6,8,10,12, etc T(Si,Al) atoms. The more T atoms are forming the channel the bigger it is. Channels of a given number of T atoms are slightly different in size according to the structure in which they occur: this is due to the different flexibility of the framework in the different cases. In the figure above (AlPO-5 structure) you can see a view across a 12 MR channel. continued...