Electric charges are known to be responsible of nucleophilic and electrophilic
attack and in general, chemical reactivity is explained in terms of electric
charges. Organic molecules, when they react catalytically inside a microporous
material are immersed in an electric field that can modify its chemical behaviour.
In spite of this widely acknowledged principle, little has been reported on this
topic and that is why we have conducted (in collaboration with Dr. Dewi Lewis)
calculation on electric fields created by zeolites. We have been able to
demonstrate how electric fields created by the microporous framework does
influence the Brønsted acidity and have found a correlation with the square
of the OH stretching frequency as shown in the figure below.
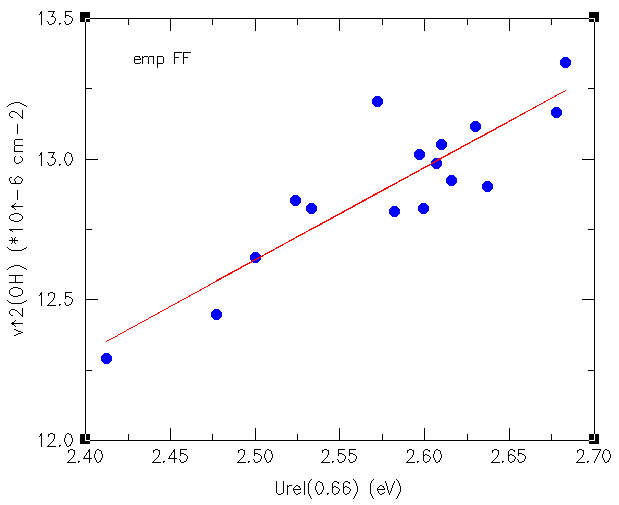
Correlation between electric field and OH stretching frequency
The circles in the figure above correspond to structures of AFI and CHA with
different chemical composition (pure silica and AlPO compositions), and also the
four crystallographic different protons are represented, so we see clearly how
independently of the source of the proton, there is a correlation between its
stretching frequency and the electric field, which is related to the energy
necessary to release the proton from its equilibrium geometry to a larger
OH distance in the direction of the OH bond.
